Drug Development: Animal Ethics vs. Protection of Man?
Zusammenfassung
Arzneistoffentwicklung muss sich heute neuen ethischen Herausforderungen stellen: Der Verbindung von Tierschutz mit dem Schutz menschlichen Lebens. Auf Basis der neuen EU Direktive zum Tierschutz analysiert der Artikel philosophische Hintergründe und Auswirkung auf die medizinische Forschung. Um Tierversuche zu ersetzen, unterstützt die EU unter anderem Alternativmethoden mit menschlichen embryonalen Stammzellen. Gleichzeitig bekräftigen EU-Deklarationen zum Schutze des Menschen in der medizinischen Forschung die unveräußerliche Würde jedes menschlichen Lebewesens. Dieses Paradoxon und seine Folgen für traditionelle Konzepte der Menschenwürde und Person rufen zum Überdenken der Mensch-Tierbeziehung auf. Eine moderate anthropozentrische Perspektive des Tierschutzes, vom thomistischen Personalismus inspiriert, könnte eine Chance für mehr Innovation in der Arzneistoffentwicklung sein.
Schlüsselwörter: Tierschutz, Schutz des menschlichen Lebens, Präklinische Arzneistoffentwicklung, menschliche embryonale Stammzellen, Europäische Gesetzgebung
Abstract
Today’s drug development is confronted with new ethical requirements: to find the right balance between animal welfare and protection of man. Analysing the philosophical background of the new EU Directive on protection of animals this article outlines major consequences for medical research. To substitute animal studies with alternative methods, the development of methods using human embryonic stem cells, among others, is supported by the EU. At the same time, EU Declarations on human rights and the protection of man in medical research affirm the inviolable dignity of every human being. This paradox – and its repercussions on traditional concepts of human dignity and the person – require a renewed reflection of the man-animal relationship. A moderate anthropocentric perspective on animal welfare inspired by Thomistic personalism may be a chance for more innovation in drug development.
Keywords: Animal welfare, human protection, preclinical research, human embryonic stem cells, European legislation
Albert Schweitzer once complained that European intellectuals keep an eye on the fact that no animals have found their way into ethics.1 Indeed, ethics in medical research traditionally have focused on the protection of the human subject and the good of the patient. The Hippocratic rule of “do no harm” to man, as expressed in the principle of non-maleficence, has represented the most important principle for medical research until recently. However, the contemporary discovery of animals in philosophical ethics and the increased awareness for animal welfare by the public requires additional responsibility and duties. Non-clinical research and drug development still relies heavily on animal studies in toxicology and safety pharmacology to predict the risk for the human subjects as well as on animal disease models to predict the efficacy of a drug candidate. Furthermore, regulatory authorities still require increasing numbers of animal studies for clinical trials and for market approval of a new drug. In addition, the development of biological drugs often requires the use of non-human primates (NHP) to prove the safety of the biological drug candidate for man.
Therefore, preclinical drug development is caught between two major demands: to protect man and to improve animal welfare. The debate is currently ongoing at all levels of society:
- At the philosophical level: In June 2011, Peter Singer, an Australian ethicist, received the “Ethics Prize” from Germany‘s Giordano-Bruno-Stiftung for his “important achievements as animal rights activist”. Singer defines some higher animals like NHP as persons, whereas newborn human beings are regarded as no persons.2 According to his philosophy, the life of a monkey is of higher value than the life of mentally handicapped human being.
- At the legislative level: A new Directive 2010/63/EU on the protection of animals used for scientific purposes,3 published on September 22, 2010, and to be applied by all EU Member States from 2013 on, strives to find the right balance between improving animal welfare and assisting research against human diseases. The new Directive focuses on the replacement of animal studies by alternative methods, the reduction of the use of animal numbers and a refinement of methods to avoid any suffering of animals during scientific experimentation.
- At the financial and research level: Through its European Research Framework Programmes, the European Commission financially supports the development of alternative strategies to animal testing such as computational biology, modelling and estimation techniques, as well as high throughput techniques and cell-based technologies.4 From 2007 to 2010, research with human embryonic stem cells (hESC), including the development of alternatives to animal studies, was supported by the EU with 21 million Euros, including 1 million Euros for a European Registry for hESC.5 Currently, consultations and evaluations are ongoing for approval of new research programmes from 2012 on.
These recent events demand an analysis of the legislative development on animal welfare in light of the following questions: What are the philosophical currents and pre-decisions which have shaped animal welfare in scientific research? Does the focus on animal welfare change the perception of the traditional values of medical research? Which values are at stake? Which factors influence the ethical decisions in preclinical drug development?
1. Current facts on animal welfare and future EU legislation
In 1986, the Council of the European Communities adopted Directive 86/609/EEC regarding the protection of animals used for experimental and other scientific purposes.6 In brief, this Directive aims to maximise animal welfare and to reduce the number of animals used in studies for scientific purposes to a minimum. Furthermore, it requires the guarantee of animal welfare in experiments, as far as general care and accommodation are concerned.7 Since the 1980s, the number of animals used for scientific experimentation has decreased significantly. This development can be observed almost in all West European nations. In Switzerland, where a large number of pharmaceutical companies are located, the number of animals used in authorized studies declined from about 2 million to about 700.000 animals during the last 25 years.8 In addition, the stress on animals has been significantly reduced using analgetics and anesthetics if procedures are painful. Animals of higher species like monkeys and dogs are only used if there are no other possibilities to show the benefit and risk for the patient. This is especially the case for biotherapeutics. Furthermore, almost all large pharmaceutical companies and contract research organisations (CROs) have local animal ethics committees in place as well as Animal Welfare Officers who are in dialogue with researchers.9 In addition, almost all European countries require a national or regional review of animal studies. Furthermore, especially US-based companies, volunteer to be accredited by the AAALAC – the Association for Assessment and Accreditation of Laboratory Animal Care International – which is a private, non-profit organisation that promotes the humane treatment of animals and inspects the companies every second year.10 The new Directive 2010/63/EU, which will replace Directive 86/609/EEC in 2013, has as its goal the harmonization of animal welfare for animals used for scientific purposes in all EU countries. Whereas countries like Great Britain, Germany and Austria already have strict animal welfare regulations in place, southern and eastern countries are well behind these requirements.
In the new Directive 2010/63/EU, animal care is based on the principles of “replacement, reduction and refinement” which were first published by W. M. S. Russell and R. L. Burch in 1959.11 These principles are also called the “3 R’s”, which are: replacement of animal studies by alternative methods, reduction of the number of animals used in an experiment and refinement of techniques used in order to decrease the incidence or amount of animal pain and distress. Therefore, as a major point, the new Directive stresses that more efforts are needed to devise alternative methods: The legislation is presented as “an important step towards achieving the final goal of full replacement of procedures on live animals for scientific and educational purposes as soon as it is scientifically possible to do so”.12 All Member States must ensure that whenever an alternative method is recognised by Community law, it is used instead of animal testing. Increasing funding for projects aiming to replace, reduce and refine the use of animals for scientific experiments is provided by the Community Framework Programmes for Research and Technological Development.13 If animal studies cannot be avoided, the choice of the species is crucial – especially when the highest developed animal species, NHP, are intended for scientific experiments.
Therefore, a second important topic deals with the use of fewer NHP. A ban proposed on using great apes such as chimpanzees and gorillas for scientific testing was generally endorsed by committee members. But the measures would have also restricted the use of other NHP such as macaques and would have hampered European scientific research on, e.g., neurodegenerative illnesses such as Alzheimer’s and chronic autoimmune diseases especially when biologics are developed.14 As a consequence, the Directive allows the use of such NHP only if there is scientific evidence that the goal of the test cannot be achieved without using these species and that it is essential for the benefit of human beings.
The use of NHP is also a very sensitive topic as these animals have a high capacity to experience pain, suffering and distress. Consequently, the new legislation will introduce categories of pain inflicted during a test (“non-recovery”, “mild”, “moderate” or “severe”). To avoid repeated suffering, the Commission proposed to allow the same animals to be reused only if the previous test entails pain classed as “up to moderate”. Furthermore, the new Directive also covers the foetuses of mammals especially in their last third of the period of development to avoid the experience of pain, suffering and distress. An upper limit of pain is required and death as an end-point of a study should be avoided.15
It is precisely the sensitivity of the animal to experience pain and suffering that is one of the most important ethical concerns related to animal studies. When carefully reading the new Directive one will discover further key phrases which go beyond the practical requirements as mentioned above and outline the philosophical background of the legislation. The demanding environment for the pharmaceutical industry often makes it very difficult to be aware of this philosophical background.
2. The complex environment for ethical decisions in preclinical research
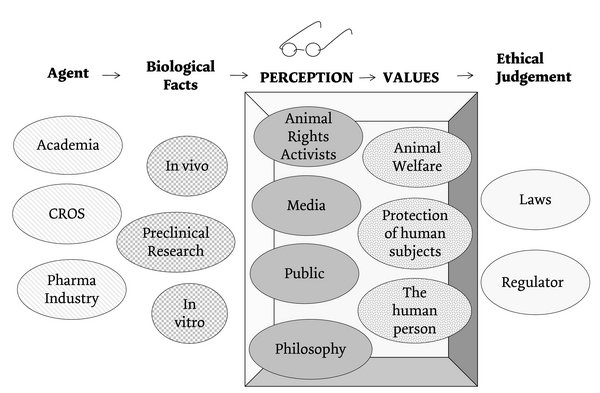
The factors which must be considered for an ethical judgement are quite diverse and complex (see Figure 1): The major agents are academia, CROs and the pharmaceutical industry. The biological facts or means to gain valid results and information for preclinical research include the in vivo and in vitro preclinical research concerning toxicological effects, pharmacological effects and pharmacokinetics. Legislations and regulatory guidelines heavily restrict and control the process of preclinical drug development especially concerning drug safety. In this context it may be of interest to note that the new Directive 2010/63/EU will influence the revision of ICH (International Conference on Harmonisation) guidelines.16 The importance of animal welfare is also reflected in the ISO (the International Organization for Standardization) 10993 Guidelines for the development of medical devices, especially Part 2 on animal welfare requirements.17
It, however, depends on the perception, how one looks at preclinical research and which values are included in one’s consideration, which represent a critical step favouring an ethical judgment on preclinical research. According to Thomas Aquinas, one can only find out the truth when reason and fact are in agreement with each other, meaning that one observes the things as they are in reality: Veritas est adaequatio rei et intellectus.18 J. Pieper calls this “seinstreues Gedächtnis”.19 But this pre-requisite is also the most endangered one, as the truth of reality is often masked by interests. The legislative and pharmaceutical industry perception on preclinical research is often shaped by hidden pre-decisions that one is not even aware of. These pre-decisions are like coloured glasses which shape one‘s point of view and analysis of values. As just indicated above, the Directive’s perception is influenced by the general public, animal rights activists, media and, finally, philosophical currents. These pre-decisions decide to which extent special values will be included into the ethical judgement on preclinical research. Will it just be animal welfare? What role does the protection of human subjects play? Do animals have rights and dignity similar to man? Can animals be persons? Are alternative methods always an ethical solution?
3. The perception and pre-decisions
When we observe animals, we are inclined to care of, respect and even love the animal. We disapprove of any cruelty in our feelings. In a further step, our reason/intelligence submits our inclination to an analysis of values, which may be of biological, psychological and cultural reasons. But instead of being open for the fullness of being and the whole good of reality, the question of animal welfare and the status of the animals is influenced by philosophical pre-decisions, which have their roots on the one hand in utilitarian approaches and materialistic evolutionism, and on the other hand in a misinterpreted anthropocentrism which results in irresponsible actions against animals.20
For anyone who is new to animal ethics, it might be quite surprising that the subject of the moral status of animals is entangled in a quite complex and fascinating philosophical debate. In the following section, I will mainly focus on views that obviously influenced the new EU legislation.
3.1 Only animal welfare and liberation
The philosophers, who can be called the “mid-wives” of animal ethics and animal liberation movement, are mainly found in the English-speaking world.21 Their thought is based on the reasoning that both animal and man are similar in being sensitive and feeling pain and, therefore, they have equal interests or similar rights as man. The root of this thought is found in English utilitarianism.
“...the capacity of animals to sense and express pain, suffering, distress and lasting harm”22
In the year of the French Revolution, Jeremy Bentham, the founder of modern utilitarianism, said that analogous to the abolishment of slavery, animals should also have rights. His hope was summarized in his famous sentence: “The question is not, Can they reason, Can they talk? But, Can they suffer”.23
“Non-human primates, dogs and cats should have a personal history file...”24
Peter Singer, the most prominent utilitarian advocate of animal liberation, continues Bentham’s thought. According to him all sentient beings have the right for equal consideration of their interests. Discrimination of any sensitive species has to be avoided. “To avoid speciesism we must allow that beings that are similar in all relevant respects have a similar right to live – and mere membership in our own biological species cannot be a morally relevant criterion for this right.”25 Therefore, Singer views animals on equal moral terms with humans. However, as important as the consideration of pain and intrinsic value of animals is for animal welfare, Singer’s philosophy on “speciesism” has repercussions on human rights. In his books “Animal Liberation”, he reasons that “there will surely be some nonhuman animals whose lives, by any standards, are more valuable than the lives of some humans. A chimpanzee, dog, or pig, for instance, will have a higher degree of self-awareness and a greater capacity for meaningful relations with others than a severely retarded infant or someone in a state of advanced senility”.26 According to Singer some animals are persons whereas some humans are non-persons.27 Singer combines here his reasoning on the equality of human and non-human animals with a concept of a human person which requires certain capacities for being regarded as such and therefore for having human rights.28 In many cases, the relevant moral capacities as mentioned above may be more developed in some animal foetuses than in human foetuses. The practical consequences for the ethical justification of animal studies are the following: Either all animal experimentation with higher developed mammals must be stopped or we have the same right to perform human trials with small children and retarded or senile humans as with animals.29 A further consequence of this current of thought is setting up alternative methods to animal studies using human embryonic stem cells supported by the European Community.30
“Animals have an intrinsic value which must be respected”31
In contrast to the utilitarian thought of equality of interests, the American ethicist Tom Regan adds another point to the ethics of animal liberation – the pro-rights argument. He continues Henry Salt’s thought, whose “Animals’ Rights” was published in 1892.32 The key concept of Regan’s philosophy is inherent/intrinsic value. This is a value that is independent of any use that it may have to others and is attributed to every creature that possesses life that matters to it.33 Many animals have something that he calls preference autonomy, which is to have preferences and the ability to initiate action with a view to satisfy them. When life matters to oneself or a being we must speak of a being as a subject-of-a-life. The subjects-of-a-life are those “beings with a biography, not merely a biology”. They “are somebodies, not somethings”.34
This includes having desires, memory, sense of the future, feelings of pain and pleasure. Every individual who is the subject-of-a-life has moral rights. What rights do animals have? They must be treated respectfully and it is unacceptable to use them as means for the goal of others. This right is an unacquired right and entitles an animal to the basic right not to be harmed. If Regan’s view gains recognition by society, it will not only prohibit the harming of animals for the purposes of scientific research, but also for recreation or the production of food and clothing.35
“The use of great apes, as the closest species to human beings with the most advanced social and behavioural skills, should be permitted only for the purposes of research aimed at the preservation of those species…”36
One philosophical thought which is often used in the debate is the argument from marginal cases. This argument stresses that humans and animals have overlapping capacities (cognitive and sensitive) and therefore we must either include at least some animals in the moral community or exclude some humans.37 Mark Rowlands writes that, “[t]he argument from marginal cases provides us with a powerful argument that applies to almost any suggested morally relevant difference between humans and animals. Whatever feature is proposed, ask yourself: ‘Do all humans have it?’ If they don’t, then ask yourself: ‘What about those humans who don’t have it?’ This argument … rules out most of the suggested relevant differences between humans and animals”.38 The argument above reflects Darwin’s view that the differences between man and animal are differences not of kind but only of degree. However, quite surprisingly, although his respect for animals is quite evident, he believed that animal experimentation was necessary for the progress of science.39 Nevertheless, Darwin represents a dividing line between pro-liberation and anti-liberation philosophers.
3.2 Man as the only moral agent: the protection of human subjects
“…the use of live animals continues to be necessary to protect human and animal health and the environment.”40
The contemporary philosophers who argue against animal liberation and the animal rights argument draw heavily from Kant’s philosophy. Kant says that human beings can have only indirect duties to animals because only human beings are ends-in-themselves as they are autonomous and can rationally consider different courses of action.41 Only human beings are moral agents. Animals cannot be moral agents. However, one must avoid being cruel to animals because those who treat animals callously are more likely to treat man without respect. The Contractarian theory by contemporary philosophers Jan Narveson and Peter Carruthers says that rights and duties need the principle of reciprocity.42,43 Morality can be only an agreement among rational, self-interested individuals. Animals cannot share an agreement with us, because they are not rational agents. On the question of how human beings like children or the senile elderly should be treated, Narveson answers that human beings have a personal interest to treat old people and children well because they will one day end up like them or because they want to avoid raising children who will be nasty adults.44 Like Kant, Peter Carruthers maintains that animals have only indirect moral significance because the way we treat them shows something about our humanity.45 Carl Cohen, another philosopher in Kant’s tradition, stresses that human beings have an obligation to treat sentient animals humanely but never owe the same moral concern to animals as to humans.46 Bonnie Steinbock concludes that humans have capacities as moral autonomy and the desire for self-respect that animals don’t have. Therefore, freedom from pain and disease is a necessary condition for the exercise of the human capacities that enables a fulfilled human life. For that reason, we are justified in making animals suffer in animal experimentation if that is the only way to free humans from pain and disease.47 This thought of balance is reflected as an essential element in the new EU guideline.
“The likely harm to the animal should be balanced against the expected benefits of the project.”48
Indeed, despite the complex philosophical dispute many philosophers now agree that animals are at least of direct moral concern and overriding the interests of animals can be only justified for important human interests.49 In contrast to hunting and eating meat scientific research is regarded as “a lifeboat situation” where we have to decide if we or the animals should suffer. Since human beings would be harmed more by suffering than animals, animal experimentation can be justified.50
4. Human embryonic stem cells instead of animals- the ethical solution?
The animal-rights movement requires the total replacement of animal studies. This is reflected in the EU Directive’s promise that,
“this Directive represents an important step towards achieving the final goal of full replacement of procedures on live animals. … The Community Framework Programmes for Research and Technological Development provided increasing funding for projects which aim to replace, reduce and refine the use of animals in procedures”.51
The European Community supports the Sixth and Seventh European Research
Framework Programmes (FP6 and FP7) which look for alternatives to replace animal testing by cell-based technologies, integrated testing strategies, bioinformatics, computational biology, computational modeling and estimation and high throughput techniques.52 Several European Organisations have the task to support, coordinate and validate alternative methods. These are the Institute for Health and Consumer Protection, the European Centre for the Validation of Alternative Methods (ECVAM), The European Partnership for Alternative Approaches to Animal Testing and the European Consensus-Platform for Alternatives. Thereby, ECVAM will play a critical role for the validation of in vitro methods which will further influence regulatory and ICH guidelines.53 Many alternative methods (like cell-based technologies and computer modelling) are quite impressive and will definitively be helpful to replace and reduce animal experimentation.
However, there are several projects in the Framework Programmes FP6 and FP7, supported by the European Community on an ongoing basis, which develop alternatives for replacing animal studies by developing tests with human embryonic stem cells (hESC) from supernumerary embryos from in vitro fertilization. In 2007, the European Commission decided to build up a European Registry for hESC, which is sponsored by 1 million Euros from FP6.54 Although Austria, Malta, Slovakia, Poland and Lithuania voted against hESC research, they must support the project financially because of the EU decision.55
In the following list, some of the 18 EU research projects using hESC (supported by 21 million Euros from the European Commission) which focus on the development of alternative methods to animal studies are summarized: VITROCELLOMICS is a predictive drug testing by human hepatic in vitro models derived from hESC.56 The aim is to deliver in vitro models that could be used by the pharmaceutical industry to replace the use of animals in investigations on liver toxicity, drug metabolism, uptake and efflux properties of compounds in the drug discovery and development processes. Especially for the risk-assessment of drug-drug interactions based on Cytochrome P450 metabolism for regulatory purposes, the in vitro test is suggested. Further projects include testing of cardiovascular effects by human cardiomyocyte in vitro models derived from embryonic stem cells (INVITROHEART)57 and ESNATS developing a battery of toxicity tests using mostly hESC.58,59 The January 2011 Progress Report on alternative methods for reproductive toxicity testing mentions that hESC are used for the assessment of developmental toxicity by several companies.60 The hESC is suggested as a test system more closely related to the in vivo situation but is still far away from being validated.
In the US, a company focused on discovery, development and commercialization of molecular biomarkers for improvement of drug safety combines hESC and metabolomics. Recently, the company announced a partnership with a European contract research organisation (CRO) which provides toxicological animal testing.61 This example indicates the pressure on pharmaceutical industry to provide alternatives to animal studies. Alternatives like the above mentioned one are suggested by CROs as an ethical solution to animal experimentation.
In April 2011, consultations started for financial support for EU research projects. During the development of the new programmes of research there will be a chance to re-evaluate research with hESC.62 This will be an opportunity for the member states to pay attention to several paradoxes:
- A paradox concerning European legislation: Article 6 of Directive No. 1982/2006/EG of the European Parliament and Council allows the financial support of research with hESC by EU in the context of FP7, whereas the European Commission declares on December 30, 2006, that research projects which involve destruction of embryos for the development of hESC will be excluded from financial support. However, the exclusion of this step of research will not prevent financial support of subsequent steps involving hESC.63
- A paradox concerning European integration of Member States: Although some Member States prohibit research with hESC or vote against it during ethical evaluation of research projects, the taxpayers of these Member States must nevertheless support financially projects with hESC.64
- A paradox concerning the protection of human subjects: Declarations and Conventions regarding medical research request the protection of each human being.65,66 But for the purpose of protecting some human beings, other human beings – human embryos – are legally allowed to be used as testing material.
- A paradox concerning human dignity, the person and human rights: Does the importance of respecting animal welfare outweigh human dignity although almost all legislation on medical research refer to human right and dignity? Comparing the regulations of English-speaking countries regarding animal welfare and protection of the human embryo confirms this (see Table 1). The points above outline the ethical dilemmas of the preclinical drug development which tries to comply with the requirements for the protection of human subjects and animal welfare.
| Country | Animal welfare regulations | Creation of human embryos for procurement of hESC67,68 | Image of man - Man-animal relationship |
|---|---|---|---|
| UK | Very strict | Allow creation of embryo for research | J. Bentham; Utilitarianism; origin of animal liberation |
| Australia | Strict | Allow creation of embryo for research | P. Singer; man and animal can be persons |
| USA | Strict | Allow creation of embryo for research | T. Regan; animal rights; principles for human protection |
| Austria | Strict | Prohibit embryo research | Human dignity, indirect duties to animal |
| Germany | Strict | Prohibit creation of embryo for research | Constitution, Nuremberg Trial, I. Kant; indirect duties to animal |
| France | Moderate | Prohibit creation of embryo for research | Human dignity, personalism |
| Italy | Moderate-low | Prohibit creation of embryo for research | Human dignity, personalism |
| China | Low | Allow creation of embryo for research | Benefit to Society > Individual |
5. The dilemma of preclinical drug development: between animal welfare and human protection
As summarized in Table 1, regulations concerning animal and hESC use for research are strongly influenced by national perception on man and animal. And these perceptions are again influenced by historically grown philosophical currents. Over time these philosophical currents have influenced the meaning of words. As nations are linguistic communities the new language constitutes also national and EU law.
However, it seems also that over time we have lost the context of many ethical key expressions and we now have only fragments of a conceptual scheme for ethics. A. MacIntyre compares this situation in his book “After Virtue” with an imaginary situation after environmental disasters where science was destroyed and only fragments of science are still known.69 Many of the meanings presupposed would have been lost and the expressions used would be arbitrary and subjective. A comparison of key expressions used in the new EU Directive for animal welfare and ethical recommendations made by the European Group on Ethics in Science and new Technologies for hESC use in research reflects well how this loss and arbitrary change of meaning shapes language. Examples of key expressions are summarized in Table 2.
| Animal70 | Human embryo / hESC71,72 |
|---|---|
| Killing | Destroying |
| Intrinsic value must be respected | Human dignity may not be maintained |
| A personal history file | “no neutral object” |
| Of great public concern | Promote public governance |
| Concern for foetal forms of mammals | Concern for the donor and health care justice |
For animals, the expression “killing” is used whereas EU documents speak of “destruction” of the embryo. Reading the documents in parallel gives one the impression that whereas animals must be respected always as living beings, human embryos at the blastocyte stage are just things with no special value. To “respect the intrinsic value of the animal” one may be allowed “not to maintain human dignity”.73 The new EU directive on animal welfare requires “a personal history file” for NHP, cats and dogs.74 But in the Recommendations on the ethical review of hESC FP7 research projects of 2007 the ethical concern on hESC is based on the fact “that human embryos are not ‘neutral’ objects”.75
These key expressions reflect the ethical dilemma of the preclinical research and drug development. Its first and most important aim is to predict the risk for the human patient and to guarantee the protection of human subjects in clinical trials. The protection of human subjects implies that man has dignity. Indeed, almost all declarations concerning the protection of human subjects in medical research from the Declaration of Helsinki76 to the Convention on Human Rights and Biomedicine77 refer to inviolable human dignity. In these declarations human dignity is meant as inalienable or intrinsic dignity present by virtue of membership to the human species regardless if the human being has certain capacities as self-awareness, social or intellectual skills. As the embryo also in the blastocyst stage is an individual human being, it has also intrinsic dignity with the same right like a human person not to be harmed. However, with the Anglo-Saxon utilitarianism and animal liberation movement, the meanings of dignity, person and intrinsic value started to change and attributed to other living species and applied in an arbitrary sense. According to P. Singer, some human beings are non-persons whereas some animals are persons. The terms person and dignity can be attributed by others and are dependent on capacities as self-awareness, social and intellectual skills and sentience. Therefore, the conclusion is that animals as dogs and NHP have extrinsic dignity, whereas human embryos and mentally handicapped new-born human beings have no extrinsic dignity.78 The consequence is that the substitution of animal studies by in vitro tests with hESC is regarded as an ethical solution according to the “extrinsic dignity” perspective. But this “ethical solution” is quite a slippery slope. As this dignity is dependent on certain capacities somewhat arbitrarily related to dignity, why should not handicapped, terminal ill or senile people substitute animals in some studies in the future?
In this context, one should remember that strong support of human dignity comes from recent emphases on international human rights for medical research and the Nuremberg trials after the Nazi’s unparalleled medical atrocities. It should be noted that in 1933, the Reichstierschutzgesetz in Nazi Germany determined that animals must be protected because of their intrinsic/inherent value. For the first time, animal studies were only allowed under certain conditions. The official Nazi ideology included respect for animals and was sensitive to animal suffering. Hitler originally planned to forbid all animal experimentation. The pressure to be competitive in medical research in Germany prevented this plan from being carried out.79 In addition, between 1933 and the end of World War II, Japanese researchers killed thousands of humans in medical experiments. Most of the researchers involved were never brought to trial. Instead, the United States got secret information from the results of Japanese biological warfare experiments and the researchers made prestigious careers.80 The Japanese called their human experimental subjects “apes”,81 whereas some victims in German concentration camps called themselves “The Rabbits”.82 One may ask if the thousands of human embryos used for human embryonic stem cell research are not today’s victims instrumentalised to promote the career of some researchers? Indeed, the EGE explicitly warns against the instrumentalisation of human life, when it notes in its recommendations on the ethical review of hESC FP7 research projects: “The creation of embryos for the sole purpose of research represents a further step in the instrumentalisation of human life.“ However, it considers spare human embryos from in vitro fertilization as an ethical alternative source to animal experimentation.83
The exclusive focus and pressure on animal welfare and reduction of animal numbers may also endanger the risk/benefit assessment for first-in-man doses. For statistical analysis, sufficient numbers of animals must be used to get meaningful results and conclusions concerning the safety margin for human subjects. Furthermore, up to now, no in vitro system is capable of predicting on its own the safety and efficacy of human subjects and is not able to substitute a whole organism. Therefore, the new EU Directive’s final goal of full replacement of procedures on live animals in medical research is a utopia. Similar to the hype concerning human embryonic stem cell research promising a cure for diverse diseases in the near future.84 in vitro tests with hESC will turn out not to be suitable to substitute all animal studies for regulatory purposes. Tests with hESC will be limited to the screening of drug candidates. New consultations for the next EU framework program are currently ongoing which may lead to a new judgment of hESC research.85
There may be also a chance for new judgments on the basis of the new animal welfare requirements at least in two areas:
- A philosophical chance: A revision of the anthropocentric relationship between man and animal as an alternative to animal rights theory, utilitarianism and contractarianism.
- A practical chance: A revision of the strategy of drug development in the pharmaceutical industry based on a harm/benefit analysis by the new EU Directive.86
6. Animal welfare as a chance for a renewed moderate anthropocentric perspective and drug development
All philosophical foundations concerning animal welfare suggest a deeply rooted respect for nature. God is substituted by Nature.87 At stake is our traditional anthropocentric conception of man as the pinnacle of the universe when “equality of animals and man” is postulated. However, Thomas Aquinas, who is deeply rooted in the anthropocentric tradition of the Middle Ages, writes: “Each error about creatures results in wrong knowledge about the creator and leads man’s spirit away from God”.88 It seems that Western industrialisation, where nature has only been playing a marginal role in recent centuries, deeply misinterpreted the original anthropocentric tradition. According to this traditional view, man has the duty to act within the order of creation and never misuse his uniqueness in the universe for domination. Currently, we are lost – not just in our irresponsibility with animals, but also in our irresponsibility with Nature and all future generations. We want a cheap price for eggs but have forgotten about the value of the chicken by getting used to the terrible housing conditions of industrial food production.
The document on Prospects for Xenotransplantation of the Pontifical Academy for Life states: “there should be a reaffirmation of the right and the duty of man … to act within the created order … in order to achieve the final goal of all creation … The sacrifice of animals can be justified only if required to achieve an important benefit for man. … However (in every) case there is the ethical requirement that in using animals, man must observe certain conditions: unnecessary animal suffering must be prevented; criteria of real necessity and reasonableness must be respected; genetic modifications that could significantly alter the biodiversity and the balance of species in the animal world must be avoided”.89 But the document also affirms that man transcends all living beings when it says that “it is man who has always directed the realities of the world, controlling the other living and non-living beings according to determined purposes”.90 This reflection of the documents is also based on Thomistic personalism according to Boethius` definition: “Persona est naturae rationalis individua substantia”.91 This implies that man has an inalienable and intrinsic dignity which is rooted in his rational nature. Between man and the rest of creation exists a gulf precisely because of his rational and spiritual nature which finds its expression in the person’s freedom, creativity, self-consciousness and interiority.92 Only man can be the subject of ethical responsibility. Only the human person can be object and subject at the same time. Whereas objectivity of persons is connected to the assumption of reducibility of the human being to the world, subjectivity means “that the human being’s proper essence cannot be reduced to and explained by the proximate genus and specific difference. Subjectivity is, then, a kind of synonym for the irreducible in the human being”.93
In addition, J. Maritain, who contributed to the drafting of the United Nations Universal Declaration of Human Rights in 1948, says that personality “signifies interiority to self”.94 This means that “the person differs even from the most advanced animals by ‘a specific inner self, an inner life’ which revolves around truth and goodness”.95 Jane Goodall, who studied chimpanzees in Tanzania almost all her life, says that the main difference between man and chimpanzees lies in the explosive development of the human intellect which is especially evident in our human language. In contrast to man chimpanzees do not ask for the sense and truth of life.96 Indeed, man is the only animal who asks the question concerning possible life after death.97 The animal is trapped by his instincts in the presence of the moment. It has all the time of the world to perceive and observe. R. Hagencord considers the animal’s life in the presence of the moment a challenge for the modern human being. Man lost the true perception of reality and is very often only guided by intellectual pre-decisions which prevent him to recognize the challenges of the moment.98
Indeed, it is in the openness of reality where the chance for animal welfare and ethical requirements for the pharmaceutical industry has to be found:
- A chance for more time to reflect and observe before animal studies are initiated.
Today’s drug developments are heavily restricted by shareholder value and, therefore, by narrow timelines and the requirement to receive market approval as quick as possible. Studies involving animals are often started without sound scientific justification. In addition, the pressure on scientists to publish as many papers as possible results in studies with inconclusive outcome. The importance of having sufficiently high numbers of animals included in the study, for statistical analysis and the risk/benefit analysis for human subjects must be taken into account. Simple reduction of animal numbers under the pressure of animal rights activists and public figures without regard to the final goal of the project will either set at risk the human subject’s safety or require a repetition of the study – therefore enhancing the number of animals significantly. Thus, careful reflection on selection of relevant animal disease models and on possible combinations of safety/efficacy/pharmacokinetics and toxicology studies must be performed. For the ethical evaluation, not only should the possible harm of animals be assessed, but also the importance and benefit of the study for the whole project.
- A chance for a harm/benefit analysis taking into account the harm of animals and the expected benefit of the drug candidate for patients.
The new European Directive on animal welfare will require from pharmaceutical industry, CROs and research institutes a harm/benefit assessment for studies and projects including animals.99 The Federation of European Laboratory Animal Science Associations (FELASA) provides some valuable principles for a thorough ethical evaluation and review of animal experiments.100 Three key points are especially interesting to note: First, the ethical evaluation of scientific projects must take into account the overall objectives of the project. Therefore, a wide enough range of expertise is of vital importance to understand the whole of reality. Secondly, “factors for consideration” are regarded valuable. However, ethical evaluation can never be reduced to checking boxes. Ethical evaluation is not a mechanical method. Ethical review must be a dialogue and can evolve with experience.101 Indeed, drug development is more than just production of a drug. The requirements for animal welfare remind us that each decisive step of drug development is also an act that may change the life of man. There is a significant difference between acting and producing, between praxis and poesis. Whereas a simple technical activity “stays outside” of the actor himself, the act as an operatio immanens stays in the actor himself and changes his life and the life of human patients.102 Reflections and decisions on the strategy of drug development will influence not just the quality of the drug product and its benefit for patients, but also affect the selection of therapeutic areas as future goals for drug development. The new focus on animal welfare requires also some revisions for drug development strategy as a whole.
- A chance for real innovation in drug development.
Drug development is driven by the market. The current utilitarian focus on health enhancement and life-style drugs often creates a health need by “condition branding” that threatens to confuse the human subject’s well-being with the well-being of the market.103 For the development of such medications, the use of animals is not ethically justified. In addition, developing “me-too-drugs” with just minor improvements of benefits for patients using animals for experimentation is ethically questionable with respect to both animal and patient: Both animal and human life will be harmed by a drug development strategy just focused on life-style and “me-too-drugs”. This is indeed the important achievement of animal ethics: to remind us that human and animal life are connected. According to the Old Testament, both man and animal have a soul (nefäsch). This does not mean that both have reason but both have the longing for happiness and need. The Latin word anima refers to “animal”. This reminds man that life is a gift and ties his happiness to all other creatures.104 Man experiences real happiness when he can accomplish his creative and innovative powers.105 Therefore, more courage for innovation is required to develop drugs which are urgently needed in the Third World – which are life-saving or are indicated for rare diseases, or which improve the life of chronically diseased patients.
The only ethical answer to today‘s animal welfare requirements is courage – to find new creative ways in drug development rather than relying on anxious, re-active actions in response to the pressure of animal rights activists. The special freedom that man has from the instinctive re-activity that animals have is also his responsibility: But without knowing about his intrinsic dignity, no ethics exist which will lead to either the happiness of man or animals.
Referenzen
- Schweitzer A., Gesammelte Werke, Grabs R. (Ed.), München (1974), p. 263: “Wie die Hausfrau, die die Stube gescheuert hat, Sorge trägt, daß die Türe zu ist, damit ja der Hund nicht hereinnkomme und das getane Werk durch die Spuren seiner Pfoten entstelle, also wachen die europäischen Denker darüber, daß ihnen keine Tiere in der Ethik herumlaufen.“
- Menschenverachtend? Kritik an Ethik-Preis für Peter Singer, Die Presse, 16 June 2011
- The European Parliament and The Council of The European Union, Directive 2010/63/EU of the European Parliament And of the Council of 22 September 2010 on the protection of animals used for scientific purposes, Official Journal of the European Union (2010); L 276: 33-79
- European Commission – Directorate General for Research Life Sciences, Genomics and Biotechnology for Health, Alternative Testing Strategies, Progress Report 2009, Replacing, reducing and refining use of animals in research, European Communities, Belgium (2009)
- Europäische Kommission richtet Stammzellen-Register ein, Europa Press Release Rapid, 29 March 2007, europa.eu/rapid/pressReleasesAction.do;
- The Council of the European Communities, Council Directive 86/609/ EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes, Official Journal of the European Union (1986); L358, 18/12/1986: 1-28
- European Commission – Directorate General for Research Life Sciences, see Ref. 4
- Interpharma, VIPS and SGCI, Pharmaforschung- Ohne Tierversuche keine Medikamente, Themendossier Gesundheitspolitik (2009); 4/09: 1-8
- FELASA-Federation of European Laboratory Animal Science Associations, Principles and practice in ethical review of animal experiments across Europe. A report prepared by the FELASA Working Group on Ethical Evaluation of Animal Experiments (2005), December 2005, pp. 1-36, here p. 36, www.felasa.eu/recommendations
- Association for Assessment and Accreditation of Laboratory Animal Care International, www.aaalac.org
- Russell W. M. S., Burch R. L., The Principles of Human Experimental Technique, Methuen, London (1959)
- The European Parliament and The Council of The European Union, see Ref. 3, 34
- European Commission – Directorate General for Research Life Sciences, see Ref. 4
- De Girolamo F., Limiting animal testing without hindering scientific research, Press release, Committee on Agriculture and Rural Development (2010), 12 July 2010
- The European Parliament and The Council of The European Union, see Ref. 3, 34, 35, 42
- Olejniczak K., European regulatory authorities: Critical updates to guidelines, implications moving forward and thinking behind new guidance, Outsourcing Preclinical Development, Berlin (2010), 1-2 December 2010
- The International Organization for Standardization, ISO 10993-2 Biological evaluation of medical devices- Part 2: Animal welfare requirements, ISO copyright office, Geneva (2006)
- Aquin T. von, Summa Theologiae, I, qu. 16, art. 2
- Pieper J., Das Viergespann, Kösel-Verlag KG, München (1998), p. 29
- De Dios Vial Correa J., Ethics in animal experimentation, in: De Dios Vial Correa J., Sgreccia E. (Eds.), Ethics of Biomedical Research in a Christian Vision, Proceedings of the Ninth Assembly of the Pontifical Academy for Life, Libreria Editrice Vaticana, Vatican City (2004), www.academiavita.org/english/Pubblicazioni/indice/eth_resbiom.html
- Taylor A., Animals & Ethics. An overview of the philosophical debate, Broadview Press, Toronto (2009), p. 8
- The European Parliament and The Council of The European Union, see Ref. 3, 36 (6)
- Bentham J., An Introduction to the Principles of Morals and Legislation, Athlone Press, London (1789), section 17, 1
- The European Parliament and The Council of The European Union, see Ref. 3, 36 (33)
- Singer P., Animal Liberation. The definitive classic of the animal movement, Harper Perennial, New York (2009), p. 19
- Singer P., see Ref 25, p. 19
- Singer P., Praktische Ethik, Reclam, Stuttgart (1984), p. 156
- Schockenhoff E., Ethik des Lebens. Grundlagen und neue Herausforderungen, Verlag Herder, Freiburg im Breisgau (2009), p. 53
- Singer P., see Ref. 27, p. 76ff.
- European Commission – Directorate General for Research Life Sciences, see Ref. 4
- The European Parliament and The Council of The European Union, see Ref. 3, 36 (12)
- Taylor A., see Ref. 21, p. 62
- Taylor A., see Ref. 21, pp. 67-73
- Regan T., Animal Rights, human wrongs. An Introduction to Moral Philosophy, Rowman & Littlefield Publishers, Lanham (2003), p. 80
- Taylor A., see Ref. 21, p. 73
- The European Parliament and The Council of The European Union, see Ref. 3, 36 (18)
- Taylor A., see Ref. 21, p. 27
- Rowlands M., Animals Like Us, Verso, London (2002), p. 45
- Darwin Ch., The Descent of Man, and Selection in Relation to Sex, John Murray, London (1890), p. 79
- The European Parliament and The Council of The European Union, see Ref. 3, 36 (10)
- Kant I., Lectures on Ethics, Cambridge University Press, Cambridge (2001), pp. 212-213
- Narveson J., On a Case for Animal Rights, The Monist (1987); 70: 31-49
- Carruthers P., The Animals Issue: Moral Theory in Practice, Cambridge University Press, Cambridge (1992)
- Taylor A., see Ref. 21, pp. 76-77
- Taylor A., see Ref. 21, p. 77
- Cohen C., The Case for the Use of Animals in Biomedical Research, N Engl J Med (1986); 315: 865-870
- Steinbock B., Speciesism and the Idea of Equality, Philosophy (1978); 53: 247-256
- The European Parliament and The Council of The European Union, see Ref. 3, 36 (39)
- Taylor A., see Ref. 21, p. 175
- Taylor A., see Ref. 21, p. 135
- The European Parliament and The Council of The European Union, see Ref. 3, 36 (39) und 36 (10, 46)
- European Commission – Directorate General for Research Life Sciences, see Ref. 4
- ECVAM Database Service on Alternative Methods to Animal Experimentation, ecvam-dbalm.jrc.ec.europa.eu
- European Human Embryonic Stem Cell Registry, www.hescreg.eu
- Europäische Kommission richtet Stammzellen-Register ein, see Ref. 5
- Vitrocellomics, er-projects.gf.liu.se/~vitrocellomics
- Invitroheart, er-projects.gf.liu.se/~invitroheart
- Embryonic Stem cell-based Novel Alternative Testing Strategies, www.esnats.eu
- European Commission, Alternative Testing Strategies, Progress Report 2010, Replacing, reducing and refining use of animals in research, AXLR8 Consortium, Germany (2010), p. 58, 84, 139, axlr8.eu/axlr8-2010-progress-report.pdf
- Moschallski N., Schuhmacher-Wolz U., Hassauer M., Schneider K, Final Progress Report: Thematic Review: Alternative Methods for Reproductive Toxicity testing - Revision and update of the sector Reproductive Toxicity testing of the publicly available DataBase service on ALternative Methods to animal experimentation (DB-ALM), Reviewed and approved by EC JRC, Institute for Health and Consumer Protection (IHCP), European Centre for the Validation of Alternative Methods (ECVAM), DataBase service on ALternative Methods (DB-ALM) (2011), January 2011, p. 89
- Stemina Biomarker Discovery, www.stemina.com/web/index.php
- Ramos-Ascensao J., Forschung - Stammzellen und die Finanzierung von Forschungsvorhaben in der EU, Europeinfos (2011), p. 137
- The European Parliament and the Council, Decision No 1982/2006/EC of the European Parliament and of the Council of 18 December 2006, concerning the Seventh Framework Programme of the European Community for research, technological development and demonstration activities (2007- 2013)
- Ramos-Ascensao J., Forschung- Stammzellen und die Finanzierung von Forschungsvorhaben in der EU, Europeinfos (2011), p. 137
- World Medical Association, Declaration of Helsinki- Ethical Principles for Medical Research Involving Human Subjects, 59th WMA General Assembly, Seoul, October 2008, www.wma.net/en/30publications/10policies/b3/index.html
- Council of Europe, Convention for the Protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine, Oviedo, 4.IV., ETS no. 164 (1997), conventions.coe.int/Treaty/EN/Treaties/html/164.htm
- The European Group on Ethics in Science and New Technologies to the European Commission, Recommendations on the ethical review of hESC FP7 research projects, Opinion (2007); 22: 32
- Stem Cell Policy, World Stem Cell Map; www.mbbnet.umn.edu/scmap.html
- MacIntyre A., After virtue, University of Notre Dame, Notre Dame, Indiana (2002), pp. 1-5
- The European Parliament and The Council of The European Union, see Ref. 3
- The European Group on Ethics in Science and New Technologies to the European Commission, see Ref. 67, 22: 1-143
- Commission of the European Communities, Commission Staff Working Paper Report on Human Embryonic Stem Cell Research, SEC (2003), 441: 1-96
- The European Group on Ethics in Science and New Technologies to the European Commission, see Ref. 67, 22: 1-143
- The European Parliament and The Council of The European Union, see Ref. 3, 36 (33)
- The European Group on Ethics in Science and New Technologies to the European Commission, see Ref. 67, 22: 41
- World Medical Association, see Ref. 65
- Council of Europe, see Ref. 66
- Singer P., see Ref. 27, pp. 168ff., 184ff.
- Schockenhoff E., Ethik des Lebens. Grundlagen und neue Herausforderungen, Verlag Herder GmbH, Freiburg im Breisgau (2009), p. 571, footnote 26. See also Lortz A., Die Entwicklung des deutschen Tierschutzrechts, in: Händel U. M. (Ed.), Tierschutz, pp. 129-143
- Tsuchiya T., The Imperial Japanese Experiments in China, in: Emanuel E. J., Grady C., Crouch R. A., Lie R. K., Miller F. G. , Wendler D. (Eds.), The Oxford Textbook of Clinical Research Ethics, Oxford University Press, Oxford, New York (2008), p. 31
- Tsuchiya T., see Ref. 80, p. 35
- Weindling P. J., The Nazi Medical Experiments, in: Emanuel E. J., Grady C., Crouch R. A., Lie R. K., Miller F. G., Wendler D. (Eds.), The Oxford Textbook of Clinical Research Ethics, Oxford University Press, Oxford, New York (2008), p. 25
- The European Group on Ethics in Science and New Technologies to the European Commission, see Ref. 67, 22
- Girgis S., Stem Cells: The Scientists knew they were lying?, Public Discourse: Ethics, Law and the Common Good, April 13, 2011
- Ramos-Ascensao J., see Ref. 62
- The European Parliament and The Council of The European Union, see Ref. 3, 37 (43)
- De Dios Vial Correa J., see Ref. 20
- Aquin T. von, Summa contra gentiles, II, c3, in: Hagencord R. (Ed.), Wenn sich Tiere in der Theologie tummeln. Ansätze einer theologischen Zoologie, Verlag Friedrich Pustet, Regensburg (2010), p. 2, “Jeder Irrtum über die Geschöpfe mündet in ein falsches Wissen über den Schöpfer und führt den Geist des Menschen von Gott fort.“
- Pontifical Academy For Life, Prospects for Xenotransplantation Scientific Aspects and Ethical Considerations, Liberia Editrice Vaticana , Vatican City (2001), No. 7, 8, 15
- Pontifical Academy For Life, see Ref. 89
- Boethius, De persona et duabus naturis, ch. III, PL 64 :1345
- Williams T. D., What is Thomistic Personalism?, Alpha Omega (2004);VII, No. 2:174-176
- Wojityla K., Subjectivity and the Irreducible in the Human Being, in: Woznicki A. N. (Ed.), Person and Community: Selected Essays, Catholic Thought from Lublin, Peter Lang, New York (1993), vol. 4, p. 211
- Maritain J., The Person and the Common Good, University of Notre Dame Press, Notre Dame, IN (1985), p. 41
- Williams T. D., see Ref. 92, see also: Wojtyla K., Love and Responsibility, Farrar, Straus and Giroux, New York (1995), p. 23
- Goodall J., Why it is Time for a Theological Zoology!, in: Hagencord R. (Ed.), Wenn sich Tiere in der Theologie tummeln. Ansätze einer theologischen Zoologie, Verlag Friedrich Pustet, Regensburg (2010), pp. 14-17
- Hagencord R., Vom verhängnisvollen Irrtum über die Tiere. Zum Projekt einer theologischen Zoologie, in: Hagencord R. (Ed.), Wenn sich Tiere in der Theologie tummeln. Ansätze einer theologischen Zoologie, Verlag Friedrich Pustet, Regensburg (2010), p. 26
- Hagencord R., see Ref. 97, pp. 27-28
- The European Parliament and The Council of The European Union, see Ref. 3, 36 (33)
- FELASA-Federation of European Laboratory Animal Science Associations, see Ref. 9, pp. 1-36
- FELASA-Federation of European Laboratory Animal Science Associations, see Ref. 9, pp. ii-iv
- Rhonheimer M., Die Perspektive der Moral. Philosophische Grundlagen der Tugendethik, Akademie Verlag, Berlin (2001), pp. 59-60
- Moynihan R., Henry D., The Fight against Disease Mongering: Generating Knowledge for Action, PloS Medicine (2006); 3: e 191
- Hagencord R., see Ref. 97, p. 35
- Messner J., Widersprüche in der menschlichen Existenz. Tatsachen, Verhängnisse, Hoffnungen, Verlag für Geschichte und Politik; Oldenbourg Wissenschaftsverlag, Wien/München (2002), p. 138
Dr. Margit Spatzenegger, Lic. bioethics
Scheibenbergstraße 38, Top 2.21, A-1180 Wien
Margit.Spatzenegger(at)gmx.net